Technologies
Air stripping
Sludge liquor from dewatering of digested sludge contains a high load of nitrogen, which is recycled back to the inlet of the WWTP. This return load usually contributes 10-20% of the total nitrogen load of the plant. It can be reduced by dedicated N removal in this sidestream, which can be realised by different processes. Apart from biological processes which eliminate the nitrogen into the atmosphere, ammonia can also be stripped in suitable conditions of high pH and temperature and then be recovered in an acidic solution, which can finally be used as liquid fertiliser.
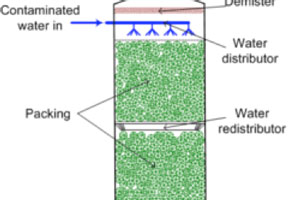
Air stripping is a proven technology which utilises ambient air to strip ammonia in a first stripper column, which is then recovered into sulfuric acid in a second column. Air stripping is typically operated at 70°C and pH 10 in columns filled with carrier material to enhance surface area for gas-liquid transfer. Air stripping is most cost-effective at high ammonia concentrations and is limited to a maximum elimination of nitrogen due to the chemical equilibrium of ammonia.
-
Purpose
-
Approach
-
Where?
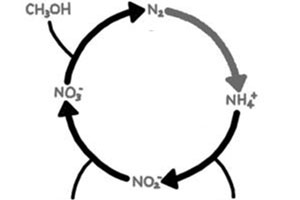
Deammonification
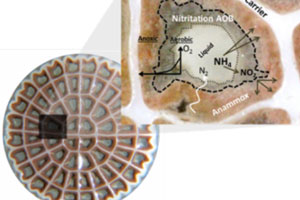
Deammonification is the process of nitritation-anammox and is a shortcut in the conventional nitrification/denitrification process. Deammonification is performed in two steps: aerobic nitritation, where slightly more than half of the ammonia is oxidised to nitrite by ammonia oxidising bacteria (AOB) and anoxic ammonia oxidation, where ammonia and nitrite are converted by anammox bacteria to nitrogen gas. Compared to conventional nitrogen removal process, no organic carbon is required for nitrogen removal and large savings of oxygen and energy demand can be achieved.
Deammonification process can be configured in one or two reactors. Performing deammonification in two reactors, where aerobic nitritation and anoxic ammonium oxidation are performed separately, gives better possibilities for optimising the growth conditions for AOB and anammox bacteria. On the other hand, deammonification in one single reactor benefits from generally lower investment costs and lower demand for operational maintenance. To allow simultaneous nitritation and anammox process, one stage deammonification is carried out in biofilms, where the biofilm can grow on carriers, or be in granules or flocs. In the biofilm, the AOB are enriched in the outer layer of the biofilm and the anammox bacteria in the anoxic inner layer of the biofilm. Specific operational conditions (pH, temperature, oxygen level) are maintained within the reactor to allow the process specific bacteria to grow in the biofilm.
Deammonification for side-stream treatment is a state of art technology, where high ammonia concentration with low COD concentration are typical characteristics of the side-stream water from dewatering of digested sludge. This reject water is usually sent back to the main treatment line, where the treatment of this ammonia load through conventional nitrification/denitrification can be quite cost-intensive. The use of deammonification process on side-stream water can dramatically reduce the nitrogen load on the existing biological treatment line. It is also a way to treat a part of the nitrogen at a low energy. In addition, since it allows a reduction of the nitrogen load on the main treatment line, it is also a solution to upgrade an overloaded existing wastewater treatment plant at a low cost.
Membrane stripping
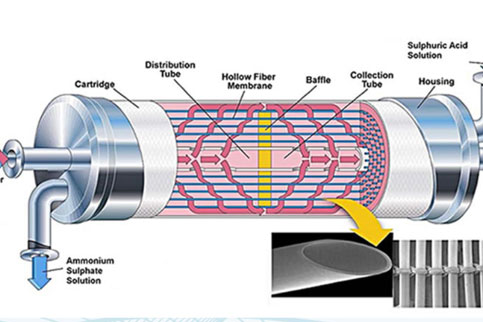
Sludge liquor from sludge dewatering is usually heavily loaded with nitrogen and contributes significantly to the nitrogen load of the WWTP. Beside biological processes for sidestream N removal (e.g. deammonification) which can have issues in process stability, N can also be removed by air, steam or membrane stripping in a physico-chemical process, thus enabling the recovery of nitrogen as a valuable liquid fertiliser (ammonium sulfate). Membrane stripping represents a compact and cost-effective method to recover the nitrogen in sludge liquors by gas-permeable membranes. To convert nitrogen into free ammonia and enable stripping, pH and/or temperature of the sludge liquor have to be increased.
Optimising pH control (e.g. by CO2 stripping combined with some caustic) and temperature conditions minimises the efforts in heat and chemical costs, and allows cost-effective operation of membrane stripping. Pre-treatment of the sludge liquor with coagulation/filtration also has to guarantee particle-free influent to the membrane in order to protect the membrane module from mechanical damage and clogging.
- Full-scale implementation of membrane stripping
- Sustec: supplier of membrane stripping plants
- BLUEtec: supplier of membrane stripping plants
- Gebr. Kunst: supplier of membrane stripping plants
Nitrification/Denitrification
Biological conversion and elimination of nitrogen in a WWTP can be achieved by the processes of nitrification and denitrification. Usually being in the form of ammonia, nitrogen in wastewater can be converted to nitrate by nitrification, which is realised by specific bacteria (“nitrifiers”) in an autotrophic process using oxygen as electron acceptor. Due to the slow growth rate of nitrifiers, nitrification requires a high sludge age to accumulate this type of bacteria in the activated sludge or biofilm. The high oxygen demand for nitrification (4.3 g O2 per g NH4-N) often defines the size of the aeration system at WWTPs.
The second step of nitrogen conversion is denitrification, which converts nitrate to gaseous nitrogen. Denitrification can be realised by many groups of bacteria, but it requires anoxic conditions to promote the use of chemically bound oxygen of the nitrate. In addition, denitrification requires a carbon source as electron donor, which is an important aspect in WWTP operation. Organic matter in the wastewater acts as carbon source, so that the ratio of bioavailable organics (COD) and nitrogen should be sufficient to guarantee full denitrification. Different configurations are available for WWTP operation such as intermittent denitrification (variation in oxygen supply over time), pre-denitrification with recycling of nitrate to anoxic tanks upstream of nitrification tanks, or cascade denitrification with multiple serial tanks of anoxic and aerobic conditions. Utilisation of microbial sludge as carbon source (“endogenous denitrification”) can also be an option, although the kinetics of this process are limited by hydrolysis of the sludge and thus require higher tank volumes. The dosing of an external carbon source (e.g. acetate) can be a last measure if residual COD for denitrification is limited.
- Nitrification & Denitrification
- Advances in wastewater nitrogen removal by biological processes: state of the art review
-
Purpose
-
Approach
-
Where?